Authors: Thomas Manaugh, Saïd Majdi, Irene Iscla
What is Alzheimer’s Disease?
The most common form of dementing illness, Alzheimer’s disease, is a progressive, degenerative disease that attacks the brain, causing impaired memory, thinking, and behavior. A person with Alzheimer’s may experience confusion, personality and behavior changes, impaired judgment, difficulty finding words, finishing thoughts, or following directions. It eventually leaves its victims incapable of caring for themselves.
What happens to the brain in Alzheimer’s Disease?
With Alzheimer’s, nerve cells in the part of the brain that controls memory are damaged. The cells develop neuritic plaques (clusters of degenerating nerve cells and amyloid protein), and neurofibrillary tangles (masses of twisted filaments of tau protein) accumulate in previously healthy nerve cells. The cortex of the brain atrophies. The ventricles in the center of the brain become enlarged.
What are the symptoms of Alzheimer’s Disease?
Alzheimer’s Disease (AD) is a dementing illness that leads to severe memory loss and loss of intellectual capacity. Symptoms usually occur in older adults (although people in their 40s and 50s may also be affected) and include loss of language skills such as trouble finding words, problems with abstract thinking, poor or decreased judgment, disorientation in place and time, changes in mood or behavior and changes in personality. The overall result is a noticeable decline in personal activities or work performance.
Who is affected by Alzheimer’s Disease?
Alzheimer’s Disease knows no social or economic boundaries. Women are almost twice as likely as men to be affected. The disease strikes older persons more frequently, affecting approximately 10% of Americans over age 65 and 47% of those over age 85. According to the Alzheimer’s Association (2020):
[There are] an estimated 5.8 million Americans age 65 and older who have Alzheimer’s dementia today. Official death certificates recorded 122,019 deaths from AD in 2018, the latest year for which data are available, making Alzheimer’s the sixth leading cause of death in the United States and the fifth leading cause of death among Americans age 65 and older. Between 2000 and 2018, deaths resulting from stroke, HIV and heart disease decreased, whereas reported deaths from Alzheimer’s increased 146.2%. In 2019, more than 16 million family members and other unpaid caregivers provided an estimated 18.6 billion hours of care to people with Alzheimer’s or other dementias. This care is valued at nearly $244 billion, but its costs extend to family caregivers’ increased risk for emotional distress and negative mental and physical health outcomes. Average per‐person Medicare payments for services to beneficiaries age 65 and older with AD or other dementias are more than three times as great as payments for beneficiaries without these conditions, and Medicaid payments are more than 23 times as great. Total payments in 2020 for health care, long‐term care and hospice services for people age 65 and older with dementia are estimated to be $305 billion.
Is Alzheimer’s Disease hereditary?
There is a slightly increased risk that children, brothers, and sisters of patients with Alzheimer’s Disease will get it, but most cases are the only ones in a family. Some patients who develop the disease in middle age (called early-onset) have a “familial” type (more than one case in the family). It is important to note that Alzheimer’s can only be definitively diagnosed after death through autopsy of brain tissue. Thirty percent of autopsies turn up a different diagnosis. Families are encouraged to ask for an autopsy as a contribution to learning more about the genetics of Alzheimer’s.
Can Alzheimer’s Disease be cured?
Presently, there is no definite cure for Alzheimer’s Disease. Unfortunately, there are unscrupulous individuals who market so-called “cures.” These treatments are often expensive and they do not cure Alzheimer’s. However, since dementia is such a troubling problem and because families are desperate to find help for loved ones, these bogus treatments continue to sell. Most of them have no scientific proof of effectiveness.
New Thinking from Integral Scientific Institute about the Role of Dehydration Among the Causes of Alzheimer’s Disease
Advances in science come both from discovering new facts and from thinking in new ways about known facts. For example, Albert Einstein’s Special Theory of Relativity didn’t add new data, but it better connected the dots about experimental facts that were at odds with Newtonian physics. His theory extended Newtonian physics to explain complicated facts better. In the end, it led to new ways of thinking by physicists about energy and matter (i.e., E = MC²).
Now, medical science requires new thinking to connect the dots as scientists search for the causes of Alzheimer’s Disease.
Using a systems-thinking lens offers significant advantages in hypothesizing the potential role of dehydration in AD. Most critically, it enables us to consider (a) how multiple elements function and interact within the various systems in the body and (b) how dehydration can operate in combination with other AD risk factors — whether sequentially, cumulatively, synergistically, or by way of a destructive feedback loop — to damage the brain and cause cognitive dysfunction.
Further, systems thinking provides a framework for identifying and observing causes and effects that change systems over the long term. If long-term observation reveals that dehydration is a cause of AD, it will enable early interventions -– interventions aimed at reducing the incidence and the severe and intransigent symptoms of AD that would otherwise appear many years later.
According to Senge (1994, page 6), “Systems thinking [is] a way of thinking about, and a language for describing and understanding, the forces and interrelationships that shape the behavior of systems. This discipline helps us to see how to change systems more effectively, and to act more in tune with the natural processes of the natural and economic world.”
Dehydration and Alzheimer’s
Sfera hypothesized that dehydration plays an important role in causing AD (Sfera et al., 2016), and Lauriola presented evidence supporting that hypothesis (Lauriola et al., 2018). Unfortunately, little or no relevant follow-up research has served to highlight and extend the work by Sfera, Lauriola, and their colleagues.
Articles exploring a possible role for dehydration as a cause of AD have been largely missing from the scientific literature. We searched the PubMed website (https://pubmed.ncbi.nlm.nih.gov/) for publications where dehydration was identified as a risk factor for AD. Not once did “dehydration” appear in the first 100 search results. Instead, the results from searching on ” ‘risk factor’ Alzheimer’s” included identifications of 41 risk factors other than “dehydration,” as is shown in the paragraph below. The number of multiple identifications for each identified risk factor is shown in parentheses when the number of such identifications was greater than one.
Adiposity, advanced parental age, ABCA7 genetic variants, age (9), air pollution, aluminum, low levels of circulating androgens, APOE4 (14), astrocytes dysfunction, atrial fibrillation, cardiovascular disease, carotid artery disease, depression, diabetes (7), dyslipidemia, low education (2), elevated cholesterol, female sex (2), genetic predisposition to inflammation, hearing impairment, hyperhomocysteinemia (2), hyperinsulinemia, hypertension, income inequality, inflammation, metabolic syndrome, mitochondrial dysfunction (3), obesity (2), oxidative stress (4), periodontitis (2), physical inactivity, sex hormones, sleep disruption (3), social exclusion, social inactivity, stroke, surgery with general anesthesia, chronic stress, thyroid dysfunction, traumatic brain injury (2), unbalanced gut macrobiota, and Vitamin D deficiency.
That we found no instance where dehydration was identified as a risk factor for AD provides evidence that dehydration as a risk factor for AD has received little attention in the scientific literature.
Declining Cognitive Abilities Track a Decline in the Body’s Hydration Status
Both longitudinal and cross-sectional studies of cognitive abilities in human subjects show cognitive processing speed slowly declining over time, starting in early adulthood and gradually declining until death (Salthouse, 1996). That pattern of decline in cognition mirrors another decline: A similar gradual decline is seen in the percentage of total body weight accounted for by water (Hydration for Health, 2020).
According to Hooper et al. (2015), in their study of dehydration among frail residents in a nursing home, “…thirst was a poor indicator of need to drink so that drinking must be regulated instead by habit and routine, which are easily disrupted in those with dementia. If this is true for older people, then deficits in drinking due to cognitive frailty could lead to dehydration which depresses cognition and thus drinking even further, a vicious circle.”
Our review of the scientific literature on cognitive abilities and dehydration has led us to suspect that there is a causal link between dehydration and degradation in cognitive abilities — specifically, and most importantly, the dramatic degradation in cognitive abilities that characterizes AD.
Impaired cognition can result from dehydration, as evidenced by symptoms of delirium, disorientation, and confusion (Mayo Clinic, 2020). Those symptoms overlap with symptoms of AD. This overlap suggests that dehydration can exacerbate certain cognitive impairments that are part of the picture of AD. Dehydration is a condition often found among AD patients (Jennings, 2018).
Here, we examine dehydration through the lens of systems thinking. Because water is present in virtually every biological process, every biological process in an organism is likely connected to every other process by water. That everything in a system is connected is a key principle in systems thinking (Acaoglu, 2017).
A Dynamic Reinforcing Loop Leads to Rapid Degradation
In this article, we employ systems thinking to connect the dots in accounting for the relatively rapid development of AD symptoms that occurs late in life, likely because of a reinforcing loop (a vicious cycle).
Systems thinking accommodates the consideration of multiple kinds of variables (e.g., biological, genetic, environmental, and behavioral) in accounting for AD development. It also accommodates the consideration of how those variables might interact in dynamic ways to cause AD.
In a reinforcing feedback loop, more of A results in more of B, and more of B results in more of A, etc. Feedback loops can have more than two nodes. In the example below, there are four nodes: dehydration-caused changes, AD, blunted thirst sensation, and adipsia.
Systems thinking about the causes of AD can help explain and describe complicated and dynamic relationships present in the development of AD. In systems thinking, a causal loop diagram depicts relationships where more of something will result in more, or less, of something else. Once drawn, a causal loop diagram can show feedback loops that characterize system behavior. Here, we describe with the help of an outline a system that contains a feedback loop. This outline contains an inciting injury that gives birth to the feedback loop. The first step in the outline is the inciting injury.
Step One: An inciting injury is a condition of dehydration brought about in a patient because of the chronic use of one or more common medicines that lead to dehydration. More about the inciting injury and the patient later.
Step Two: Dehydration from the inciting injury causes conditions known to lead to the kinds of degenerative structural changes and cognitive impairments that are symptomatic of AD. Clinically obvious symptoms that result from actions in Step Two do not occur immediately. Rather, they manifest increasingly over time. Importantly, the destructive actions increase in intensity over time because of a feedback loop. The feedback loop contains Step Two that then leads to symptoms of AD. Among the destructive conditions created in Step Two are reduced vascular perfusion and a reduced flow of cerebrospinal fluid to remove toxins and wastes (Weller et al., 2008).
Step Three: A feedback loop is established that leads from Step Two — to symptoms of AD — to blunted thirst sensation — to adipsia (inadequate drinking response to maintain normal hydration) — back to Step Two (dehydration leading to neurodestructive changes) — back to symptoms of AD. See Figure 1.
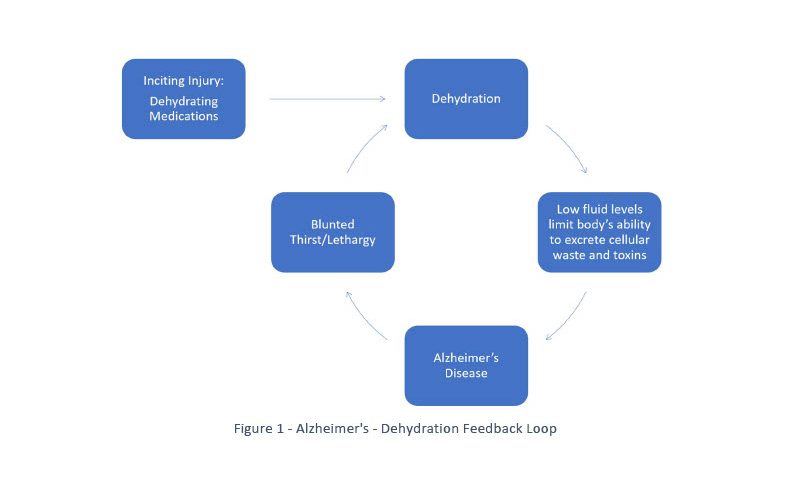
Over time, the feedback loop creates destructive effects that might become diagnosed as mild cognitive impairment (MCI). Later, absent some intervention to disrupt the reinforcing loop, increasingly severe symptoms of cognitive degradation allow a diagnosis of AD.
As a chronic condition, dehydration reaches an apogee during ages in the 70s and 80s — the same ages when AD is most likely to be diagnosed. This is not a coincidence. Rather, we hypothesize that severe symptoms of AD become manifest during older ages when symptoms of AD become part of a self-reinforcing loop that includes blunting of the thirst sensation, reduced drinking of fluids, and increasingly high levels of dehydration.
An Inciting Injury — the Role of Dehydrating Medicines
Undiagnosed AD starts as a slowly developing process of cognitive degradation that is a normal part of aging. However, an inciting injury can tip a slowly developing process into a rapidly developing, self-reinforcing loop. According to systems thinking, that increased speed of degradation signals a “step function” event. Such events may lead to an entire system becoming unstable.
We hypothesize that dehydrating medicines create an inciting injury because of cumulative effects over many years. Below are some dehydrating medicines used to treat a variety of common conditions (Puga et al., 2019).
- Diuretics are often prescribed for heart failure and sometimes for high blood pressure.
- Laxatives treat or prevent constipation.
- Antacids require moisture when absorbed by the body.
- Antihistamines can affect fluid balance.
- Non-steroidal anti-inflammatory drugs for inflammation and pain can cause diarrhea.
- Blood pressure medicines can induce diuresis.
- Antibiotics can induce diarrhea.
- Corticoids can increase urine volume.
Dehydration episodes cause shrinkage in gray and white matter areas in the brain (Streitbürger et al., 2012). Thus, dehydrating episodes that frequently occur over an extended period could be hypothesized to result in persisting neurodegenerative changes in the brain. Those changes would be in addition to other changes that might occur because of the naturally occurring dehydration that is a part of aging (Hydration for Health, 2020).
We hypothesize that numerous episodes of dehydration initiate a destructive reinforcing loop. Those episodes, caused by dehydrating medicines, constitute an inciting injury when their effects accumulate over time. Once the reinforcing loop is initiated, increasingly severe symptoms of AD begin to be seen, and neurodegenerative changes continue to occur independently from the inciting injury.
It is reasonable to suspect that dehydration caused by long-term use of dehydrating medicines would have a significant and lasting impact on the brain. Lauriola et al. (2018) pointed out how important hydration is to the structure and function of the brain:
“Dehydration has been reported to be the most common fluid and electrolyte imbalance in older adults [29–31]. Recent clinical studies have shown that the hydration state affects cognitive performance, particularly visual attention and mood [32]. The changes in extracellular osmolality inevitably affect the intracellular environment determining important alterations in the volume and function of cellular mechanisms causing irreversible morphological and functional damage.”
AD patients typically experience a marked decline in their cognitive abilities sometime after the age of 60. Viewed from a systems-thinking point of view, that rapid decline likely results from the operation within the system of a destructive reinforcing loop. This scenario occurs in countless systems that fail when vulnerable elements within a system come under stress from a self-reinforcing, destructive loop after an inciting injury.
An example of instability from another discipline occurs when an airplane’s motor mount develops the inciting danger to the injury of a crack. Suppose the weakened motor mount begins to vibrate in a reinforcing loop that causes the crack to widen. In that case, the operation of the reinforcing loop poses a danger to the stability of the entire airplane.
Many commonly used medicines are used for decades by AD patients before the time when, in their 60s or later, AD patients begin to show symptoms. Thus, a long prodromal period precedes a relatively short period during which severe symptoms appear.
Accounting for the Higher Incidence of AD among Women as Compared to Men
Identifying the high use of dehydrating medicines as an inciting injury for women is consistent with evidence that women are about twice as likely as men both to use medicines and to develop AD (Mazure, 2016).
According to Mosconi (2020, page 21), the higher incidence of AD among women is not accounted for by the fact that women live longer than men: “… [S]tatistical models that account for gender-dependent mortality rates broadly show the same 2:1 ratio at any age. In plain English, women with Alzheimer’s outnumber men with Alzheimer’s two to one regardless of their age, age at death, and difference in life span.”
According to Sherzai & Sherzai (2017), members of a community of Seventh-day Adventists in Loma Linda, California, have a low incidence of AD. They also tend to avoid the use of medicines because of their faith. We are interested in finding out more details about (a) the incidence of AD in that community compared to other groups and (b) how women in the community compare to men in the community with respect to their incidence of AD. We suspect that the disparity in the incidence of AD between women and men will be relatively small because both sexes tend to eschew the use of medicines.
Fraser et al. (1996) did not compare the incidence of AD between elderly men and elderly women from a community of Seventh-day Adventists. However, a test for cognitive impairment was administered, the MMSE (Folstein et al., 1975), which is commonly used to help in the diagnosis of AD. When MMSE scores were correlated to the sexes of participants in the study, the correlation value was not found to be significant. That resulting non-significant correlation is what one would expect if the women in the study (who presumably avoided medicines) were no more likely than the men to show signs of cognitive impairment. That result supports our hypothesis that the difference in the incidence of AD between men and women is at least partly due to the fact that women are more likely than men to use dehydrating medicines throughout their lives. We expect that neither the women nor the men in the church would have been subject to effects from the chronic use of dehydrating medicines. That is, neither group would have been subject to what we have hypothesized to be the inciting injury for AD that might result from frequent use of dehydrating medicines.
Parenthetically, we are interested in accessing the same database of medical records for Seventh-day Adventists as was used by Fraser and his colleagues. We have sent a letter of intent to the administrators of the database, asking for access to its information. The database contains medical data made available to researchers by the Church of Seventh-day Adventists and Loma Linda University (Loma Linda University Health, 2020).
Discussion and Questions for Future Research
Mosconi (2015, p.37) wrote: “… water is involved in every chemical reaction in the brain. In fact, brain cells require a delicate balance of water and minerals and salts to work at all. … Further, water is indispensable for energy production … and also helps to form proteins, absorb nutrients, and eliminate waste products.” Given the centrality of water in crucial physiological processes in the brain, it would be surprising if dehydration were not to lead to some measurable level of dysfunction.
In a paragraph above, we listed 41 different risk factors for AD. It is likely that virtually all of those identified risk factors could be either riskier or less risky, based on hydration status. For example, disturbed sleep presents a risk that the beta-amyloid protein will not be cleared from the brain while one sleeps, thereby allowing amyloid plaques to form (Winer et al., 2020). Similarly, one of the problems said to result from dehydration is that amyloid protein is more likely not to be cleared from the brain and for it to misfold to form neurotoxic amyloid plaques (Sfera et al., 2016). Both dehydration and sleeplessness are likely to be marked by symptoms of fatigue, confusion, “brain fog,” and memory lapses. It is possible, even probable, that dehydration would combine synergistically with sleeplessness; disturbed sleep would become a more potent risk factor for AD.
Lack of saliva in the mouth (“dry mouth”) is known to facilitate the growth of porphyromonas gingivalis bacteria that cause periodontal disease. Dry mouth is a symptom of dehydration. Singahroo et al. (2015) presented evidence that the spread of periodontal disease pathogens into the brain is associated with AD. It is logical to hypothesize that dehydration creates a condition in the mouth that could ultimately lead to the development of AD (Beydoun, M. et al., 2020; Singhrao et al., 2015).
The brains of seemingly normal persons have been dissected after death, only to find their brains riddled with beta-amyloid plaques (Perl, 2010). An interesting question is whether those persons might have maintained especially good habits for staying well hydrated throughout their lives. Another question is whether it takes different levels of dehydration to trigger the degradation of critical physiological functions, including brain functions.
Do the Japanese live long and have a low incidence of AD because they are relatively well hydrated due to their tradition of drinking water in the morning (Arab, 2010)? What other cultural differences in drinking water are correlated with longevity and a low incidence of AD?
Summary and Conclusions
Using facts published in the scientific literature and systems thinking, we have stated the following hypotheses about the roles of dehydration in causing AD:
- Dehydration has been largely ignored as having a role in causing AD.
- Numerous dehydrating episodes, created by chronic use of dehydrating medicines over many years, can inflict an inciting injury that creates a self-reinforcing loop that causes AD. The self-reinforcing loop can continue to operate independently of the inciting injury. It includes symptoms of dehydration and symptoms of AD. Over time, increasingly severe symptoms of AD become manifest.
- Rapid neurodegeneration because of AD late in life can be explained by the operation of the destructive reinforcing loop that includes symptoms of dehydration and symptoms of AD.
- Women are more likely than men to become diagnosed as having AD because they are more likely to experience during their lifetimes chronic use of dehydrating medicines.
- Dehydration can work synergistically with known risk factors for AD (e.g., disturbed sleep) to cause neurodegenerative changes in the brain that are symptomatic of AD.
- Though dehydration does not directly cause periodontal disease to infect the brain, dehydration does cause dry mouth – a condition known to foster growth of the pathogens that are suspected of infecting the brain and causing AD.
- The incidence of AD can be reduced in populations that maintain practices for staying well hydrated.
We are especially interested in exploring the idea that a long-term effect of some dehydrating medicines could be to incite a reinforcing loop that leads to increasingly severe AD symptoms. That is an important possibility to consider because women use those medicines more than men and also have a higher incidence of AD. Clearly, it would be a breakthrough in medical science and practice if significantly lower rates for women developing AD could be achieved by simply developing medicine-taking regimens that would be less dehydrating.
For all people, conscientiously to maintain a healthy hydration status throughout their lives is an inexpensive kind of medical prescription that might well result in a significant reduction in the incidence of AD.
References
Acaroglu, L. (2017, September 7). Tools for Systems Thinkers: The 6 Fundamental Concepts of Systems Thinking. Medium. Retrieved from https://medium.com/disruptive-design/tools-for-systems-thinkers-the-6-fundamental-concepts-of-systems-thinking-379cdac3dc6a.
Alzheimer’s Association (2020). 2020 Alzheimer’s disease facts and figures. Retrieved from https://alz-journals.onlinelibrary.wiley.com/doi/full/10.1002/alz.12068Abstract.
Arab, L., & Sabbagh, M. (2010). Are Certain Lifestyle Habits Associated with Lower Alzheimer Disease Risk?. J Alzheimers Dis. 20(3): 785–794. doi:10.3233/JAD-2010-091573.
Beydoun M., Beydoun H., Hossain S., El-Hajj Z., Weiss J., & Zonderman A. (2020). Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J Alzheimers Dis. 75(1):157-172. doi: 10.3233/JAD-200064.
Folstein, M., Folstein, S., & McHugh, P. (1975). “Mini-mental state” : A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189-198.
Fraser G., Singh P., & Bennett H. (1996) Variables Associated with Cognitive Function in Elderly California Seventh-day Adventists. Am J Epidemiol. Jun 15;143(12):1181-90. doi: 10.1093/oxfordjournals.aje.a008705
Hooper, L., Bunn, D., Downing, A., … Shepstone, L. (2016). Which Frail Older People Are Dehydrated? The UK DRIE Study. J Gerontol A Biol Sci Med Sci, Vol. 71, No. 10, 1341–1347.doi:10.1093/gerona/glv205
Hydration for Health (2020, December 8) Make healthy hydration the new norm. Retrieved from https://www.hydrationforhealth.com/en/.
Jennings, A. (2018). Detecting and Preventing Dehydration in Alzheimer’s Patients in Nursing Homes: A Case Study Approach. Journal of Alzheimer’s Parkinsonism & Dementia. Retrieved from https://scientonline.org/open-access/detecting-and-preventing-dehydration-in-alzheimers-patients-in-nursing-homes-a-case-study-approach.pdf.
Lauriola, M., Mangiacotti, A., D’Onofrio *, G … Sancarlo, D. (2018). Neurocognitive Disorders and Dehydration in Older Patients: Clinical Experience Supports the Hydromolecular Hypothesis of Dementia. Nutrients, 10, 562.
Loma Linda University Health (2020, December 8). For Researchers. Retrieved from https://adventisthealthstudy.org/researchers.
Mayo Clinic (2020, December 8). Delirium. Retrieved from
https://www.mayoclinic.org/diseases-conditions/delirium/symptoms-causes/syc-20371386.
Mazure, C., & Swendsen, J. (2016) Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. Apr;15(5):451-2. doi: 10.1016/S1474-4422(16)00067-3.
Mosconi, L. (2018). Brain food: the surprising science of eating for cognitive power. Avery, New York.
Mosconi, L. (2020). The XX Brain. Penguin Random House, New York.
Perl, D. (2010). Neuropathology of Alzheimer’s Disease. Mt Sinai J Med. Jan-Feb; 77(1): 32-42. doi: 10.1002/msj.20157.
Salthouse, T. (1996) The processing-speed theory of adult age differences in cognition. Psychol Rev. Jul;103(3):403-28. doi: 10.1037/0033-295x.103.3.403.
Senge, P. (1994). The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. New York: Currency, Doubleday.
Sfera A, Cummings, M, & Osorio, C. (2016). Dehydration and Cognition in Geriatrics: A Hydromolecular Hypothesis. Front Mol Biosci. May 12;3:18. doi: 10.3389/fmolb.2016.00018.
Sherzai, D. & Sherzai, A. (2017). The alzheimer’s solution. Harper One, New York, N.Y.
Silver A. (1990). Aging and risks for dehydration. Cleve Clin J Med. Jun;57(4):341-4. doi: 10.3949/ccjm.57.4.341.
Singhrao, S., Harding, A., Poole, S., Kesavalu, L., & Crean, S. (2015.)Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer’s Disease. Hindawi: Mediators Inflamm. 2015:137357. Retrieved from https://www.hindawi.com/journals/mi/2015/137357/ doi: 10.1155/2015/137357.
Streitbürger, D., Möller, H., Tittgemeyer, M., Hund-Georgiadis, M., Schroeter, M., & Mueller K. (2012). Investigating structural brain changes of dehydration using voxel-based morphometry. PLoS One. 7(8):e44195. doi: 10.1371/journal.pone.0044195.
Weller, R., Subash, M., Preston, S., Mazanti, I., Carare, R. (2008). Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. Apr;18(2):253-66. doi: 10.1111/j.1750-3639.2008.00133.x.
Winer, J., Mander, B., Kumar, S., Baker, S., Jagust, W., Walker, M. (2020). Sleep Disturbance Forecasts β-Amyloid Accumulation across Subsequent Years. Waters Dental Group.Volume 30, Issue 21, P4291-4298.E3, November 2. Retrieved from https://watersdentalgroup.com/category/general-dentistry/.
